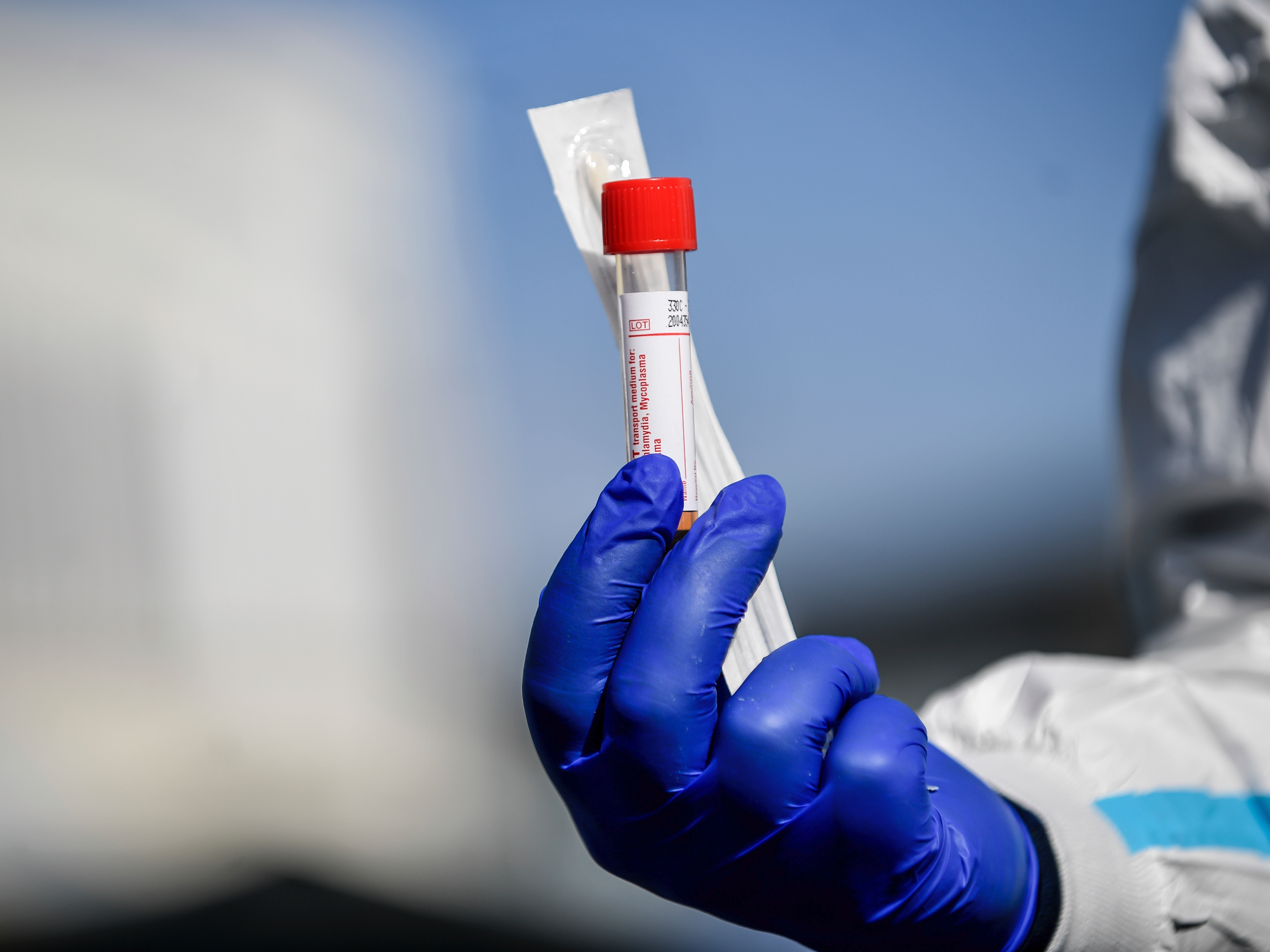
- The USDA has authorized at-home coronavirus testing kits to allow people to take samples on their own and send them in to a lab to determine if they are infected.
- The emergency-use authorization applies to tests from LabCorp only.
- The tests cost $119, and you need to complete an eligibility form to order them, but you can receive results one to two days after the sample is received by the lab, depending on demand.
- Because of limited availability, first responders and healthcare workers have priority.
- Visit Business Insider’s homepage for more stories.
As the US works to contain the spread of the novel coronavirus, testing has become a crucial part of identifying, tracking, and isolating new infections.
The US Department of Agriculture and Food and Drug Administration recently announced an emergency-use authorization for coronavirus home-testing kits, which allow people to take samples at home and send them by mail into a lab to detect a current infection.
The ruling applies to only one test from a single company, LabCorp. The company already had approval for COVID-19 tests that use swabs collected by medical professionals.
The kits are available online for $119, which includes two-way overnight shipping. To order one, however, you need to complete an eligibility questionnaire online.
Tests are available to only certain medical workers
Since the supply of testing kits is limited, first responders and medical workers have priority in ordering them, according to the LabCorp website. According to a representative, the company plans to make tests more broadly available in the near future, though a specific timeline hasn't been released.
"We expect to expand the availability of the self-collection kits to additional patients assuming adequate supplies. However, it is premature to comment further about exact timing," a spokesperson for LabCorp told Business Insider via email.
The tests also aren't available in New York, New Jersey, Maryland, and Rhode Island, since those four states have additional regulations on testing procedures.
"Emergency use" means that the test has data to support its use but has not been formally approved by the FDA and can be used only as long as the emergency circumstances continue.
Any other companies looking to offer at-home testing kits would require an additional emergency-use authorization, according to the FDA. The FDA previously cracked down on startup companies attempting to create at-home testing kits without approval.

Home tests could be more accessible and less invasive than other diagnostics but still aren't perfect
So far, the FDA hasn't authorized any coronavirus tests that are fully processed at home.
The home kits allow you to collect a nasal sample at home using a swab inserted inside the nostril. The samples then must be mailed to a lab for testing. But this procedure is far less invasive than other tests that require medical workers to insert a swab deep into the patient's nose and throat.
Self-swabbing can be just as effective as swabs administered by healthcare professional, according to recent research from UnitedHealth Group. It may also be safer, since self-swabbing protects medical staff from being in close proximity to patients and infectious particles.
Before it authorized the LabCorp test, the FDA allowed self-swabbing only in clinical settings, such as a drive-thru-testing clinic, TechCrunch reported.